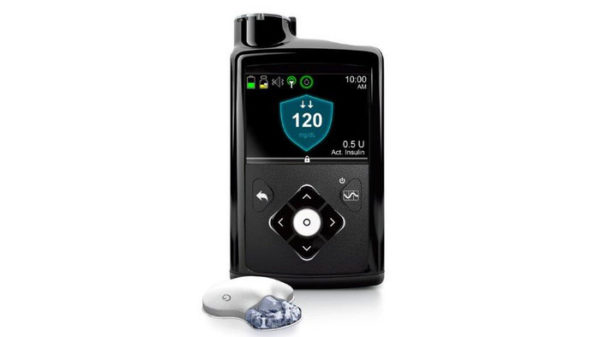
“This is a historic milestone,” said Aaron Kowalski, chief mission officer of JDRF, the organization that helps fund the research of the newly approved Medtronic MiniMed 670G hybrid closed loop system (consisting of an insulin pump and CGM (continuous glucose monitor)).
The hybrid solution removes the risk of “error-prone adjustments,” which is common when the devices operate separately.
If blood sugar levels are consistently high over long periods of time, organ damage is likely to occur, while low blood sugar levels can lead to unconsciousness. The previous model would shut down the pump if blood sugar levels dropped too low, but the MiniMed 670G predicts the drop in sugar and prevents these life threatening issues completely.
This is monumental news for Type 1 diabetics, who are reliant on their injections or pumps due to the lack of insulin production in their bodies. The approval of the MiniMed 670G offers Type 1 diabetics a new lease on life. The MiniMed is being considered the first “artificial pancreas” system.
It is labeled as a hybrid because it is not a fully closed loop: users still need to signal if they’re about to eat and estimate the number of carbs they will ingest, so the device can measure how much insulin to produce. There is still a chance for mistakes, however, the MiniMed 670G can predict a miscount and correct it automatically. Users can now sleep soundly, without worrying about their levels.
There are some challenges: insulin injected under the skin takes too long to work and these devices need a signal prior to eating. Researchers are trying to create a faster device that can deliver insulin directly without the lag time. The faster a device can work, the closer it will come to a “closed loop” system. Another issue is the device’s “wearability.” The company is trying to create an even smaller device to make it more comfortable for users. At this time, the MiniMed 670G is only approved for people ages 14 years or older, however, the JDRF is researching its effectiveness on children 7 to 13 years of age.
The financial downside of the device is that Medicare does not cover CGM technology. Users will have to give up their devices, or pay out of pocket when they reach 65 years of age. The JDRF is lobbying to change insurance coverage issues as well as educate potential users about the advantages of the new diabetes technologies on the market today.
Read the full article here.
Comments are closed.